News & Knowledge
Portable medical electronics: a new profitable mar
时间:2020-01-07 22:00:18
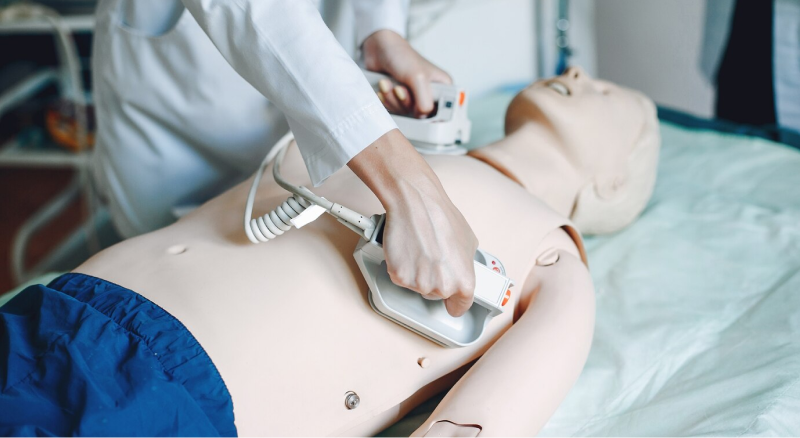
Ten years ago, it was hard to imagine that an ultrasound device weighing 150 kilograms and the size of a dressing table would be reduced to a product that could be held in the hand. But it has really come true. This portable color ultrasound device manufactured by American medical device manufacturer SonoSite has now sold more than 19,000 units worldwide.
Similar portable medical electronic devices are gaining favor in the market. This is obviously a growing market, but so far, traditional Chinese electronic OEMs and EMS have paid little attention to it. Market research company Databeans predicted in 2004 that the capacity of semiconductors in the entire medical device market would reach US$59 billion; another report pointed out that sales of wireless medical electronic devices in Europe would increase from US$98 million in 2003 to US$445.8 million in 2008. "This represents a small part of a larger market," said Keith Robinson, industry manager at Frost & Sullivan, "but it is clearly a growing market."
Two factors can also indirectly prove the above argument. First, countries around the world, especially the US government, are asking the healthcare industry to control costs. Second, some countries are aging, and the elderly are concerned about health care and demand affordable health care reforms that would not have been possible 10 years ago.
Robinson also believes that traditional medical devices are converging with digital consumer electronics and wireless products, which are shrinking in size, lighter in weight, adding more features, and connecting to wireless networks. This marks the consumerization of medical electronic devices, which are moving out of hospitals and doctors' offices and into other channels. This seems to be somewhat similar to the development trend of wireless devices.
For example, automatic electronic heart defibrillators first hit the counter in 2004. Greg Love, CEO of Micro Power Electronics Inc., said that the typical cost of a defibrillator has dropped from $5,000 to $2,000. The company provides battery systems for 70% of the defibrillator market. He said: "Mobility and portability are becoming the key factors affecting the medical electronics industry."
The trend of miniaturization of medical electronic devices began with blood glucose monitors and expanded to other devices such as wearable heart monitors and infusion pumps.
Plexus, an American company, gets 30% of its annual sales revenue from medical product manufacturing, amounting to $300 million. Brad Goskowicz, vice president of the medical division of Plexus Technology Group, an EMS provider under the company, has found that the market for home monitoring devices using radio frequency technology is rising, such as communication devices used to monitor heart patients. Goskowicz also mentioned that there are more and more types of medical electronic products that can be implanted in the human body, such as pacemakers.
At the same time, large OEM manufacturers such as GE Medical Systems and Philips Medical Systems realize that they need to reduce equipment costs and develop products faster in order to remain competitive in the portable world. In the process, these manufacturers are breaking with tradition and turning to EMS manufacturers for help.
"EMS providers can bring their expertise in multi-layer circuit board design, imaging and image processing, wireless and multi-chip modules to the medical field, allowing medical OEMs to further improve their products from a technological perspective," Robinson said. "The medical electronics industry is years behind other markets in some areas."
Rules for US market access
Manufacturing a defibrillator is obviously different from making a 3G phone. It must meet the requirements of medical and health regulatory laws and regulations. Let's first look at the rules in the United States. In the United States, although the production of electronic products is handled by EMS companies, the final assembly work is tightly controlled by medical OEMs because they must ensure the overall performance, intellectual property and compliance with the regulations of the US Food and Drug Administration (FDA).
First, the manufacturer must be an FDA-registered or FDA-approved company. FDA-registered companies are companies that have been verified to have a quality system that meets the Federal Good Manufacturing Practice (GMP) for pharmaceutical manufacturing; FDA-approved companies are those that provide products to end customers and therefore must meet further requirements. For example, an EMS company called SMC is an FDA-registered company, but not an FDA-approved company, and its OEM customers are responsible for the final testing of the product.
There are several steps to meet FDA requirements. A major step is to document medical products, from conception to, in many cases, the end of the product's life. Industry insiders say FDA requirements are not onerous, but attention to detail is more important than with consumer products because of the legal implications. "If you don't document your product well, you're going to have trouble getting government approval after manufacturing," says Dan Marinsik, vice president of quality assurance and regulatory affairs for the medical division of Four Seas.
Currently, medical electronics account for about 6% of Four Seas' total sales revenue. Marinsik says that percentage has increased by 5% per quarter over the past two years and is expected to rise to double digits by 2008.
In addition, careful measures must be taken to prevent gray market materials and components from being mixed into medical products, and suppliers must be approved by the FDA. "The FDA should be involved and review the qualifications of suppliers," Marinsik says. Four Seas itself regularly audits its suppliers and uses a global ERP system to ensure that raw materials are not sourced from sources other than the AVS list. "You always have to be legal in everything that has to do with medical," he says.
With documentation comes traceability. Once medical electronics leave healthcare facilities and doctors’ offices, component traceability becomes a critical and challenging issue.
Service records are also strict. For example, if an X-ray tube needs to be replaced, the OEM’s installed product database must also reflect the serial number of the new X-ray tube after the technician replaces it for the customer.
Service and repair are key areas. If a medical electronic device is returned to the OEM for maintenance, the entire process must be documented. “If you see a trend or a potential problem that could cause harm, you must notify the FDA,” Marinsik said.
Medical OEMs have traditionally assumed product liability, but this may change as more products are outsourced. Some EMS companies are discussing product liability issues with OEMs more in-depth than in the past. “The law is obviously something everyone should pay close attention to,” Robinson said.
Industry insiders say that despite the layers of regulation surrounding the manufacture of medical electronics, electronics manufacturers should not be intimidated. Bringing a factory into FDA compliance is not as difficult as it may seem. "Once you get in and become part of the culture, FDA-compliant manufacturing becomes part of the overall process and is not particularly troublesome," said Goskowicz of Plexus. "Its regulation will not limit or slow down the growth of the company."
But smaller electronics manufacturers may face some challenges when it comes to documentation and traceability requirements. John Zurborg, vice president of sales at SMC, said that developing a corresponding traceability management system may lead to a significant increase in costs.
Pay attention to whether the business model matches
Strong engineering design capabilities are also one of the potential difficulties. Marinsik of Sihai pointed out that the life cycle of medical electronic devices is longer than that of consumer electronics. The life of small home medical electronic devices is generally 3-5 years. Although the life cycle is longer, medical electronics products require more attention to design than consumer electronics products in terms of accuracy and ease of use.
The biggest trouble is that medical electronics products are usually produced in small batches with multiple varieties. Chinese electronics manufacturers equipped with production lines suitable for large-scale manufacturing may not be suitable for meeting the multiple varieties required by medical OEMs.
In fact, whether the business model matches is the most important criterion when evaluating EMS suppliers. For example, a top EMS was awarded a $20 million contract, but six months later found that the costs of meeting FDA requirements and rapidly changing technology were significantly different from the company's usual manufacturing methods.
Whether or not to have factories around the world is not a decisive factor in attracting medical OEM customers. Generally speaking, OEMs want final assembly to be done in North America so that they can easily send engineers to the EMS's factories on a regular basis. For example, all of Sonosite's ultrasound equipment is finally assembled in North America, and 50% of them are shipped all over the world.
Robinson also believes that production in Asia also involves intellectual property security issues. In the medical market, counterfeit goods may endanger users. But some people do not fully agree with this view. "Our customers require a more global layout, and products need to be produced close to their sales markets," said Goskowicz of Plexus. For example, circuit boards for medical products are produced in Asia, then shipped to Europe for final assembly and sold to the European Union.
In some ways, the production of medical electronics is following the model of other electronics fields. Large OEMs want flexibility when developing products, while small OEMs do not want to invest in factories. "Smaller companies often have a termination strategy, which is to wait to be acquired, and the factory will become a burden to them," Goskowicz said.
SMC's Zurborg pointed out that in a recent contract bidding, a large medical OEM specifically asked for an EMS partner with a global manufacturing presence. "This is a prime example of an OEM trying to reduce costs," he said. China and India are obviously the preferred low-cost supplier markets.
In addition, the trend of portable medical products will also prompt OEMs to hand over product manufacturing to low-cost electronics manufacturers. "Typically, OEMs will go to EMSs that focus on producing medical products," he said. "With the consumerization of medical electronics, they must go the low-cost route to remain competitive, which will bring a large number of mid-sized EMSs into the market."
Analysts generally believe that the medical electronics market will not be as prone to bubbles and bursts as other industries. But if Chinese electronics manufacturers decide to invest in the medical electronics market, they should take a cautious approach because the returns will not be immediate. But over time, the portable medical electronics market may provide more stable and lasting returns than other business areas.
Next: Realization of medical monitoring system based on
Back to list


